COVD-19 ETHICS AND REGULATIONS GUIDING THE CONDUCTION OF CLINICAL TRIALS IN DEVELOPING NATIONS
Clinical trials in developing nations and Covid 19 pandemic.
Featured Blogger
August 17, 2025
04/08/20
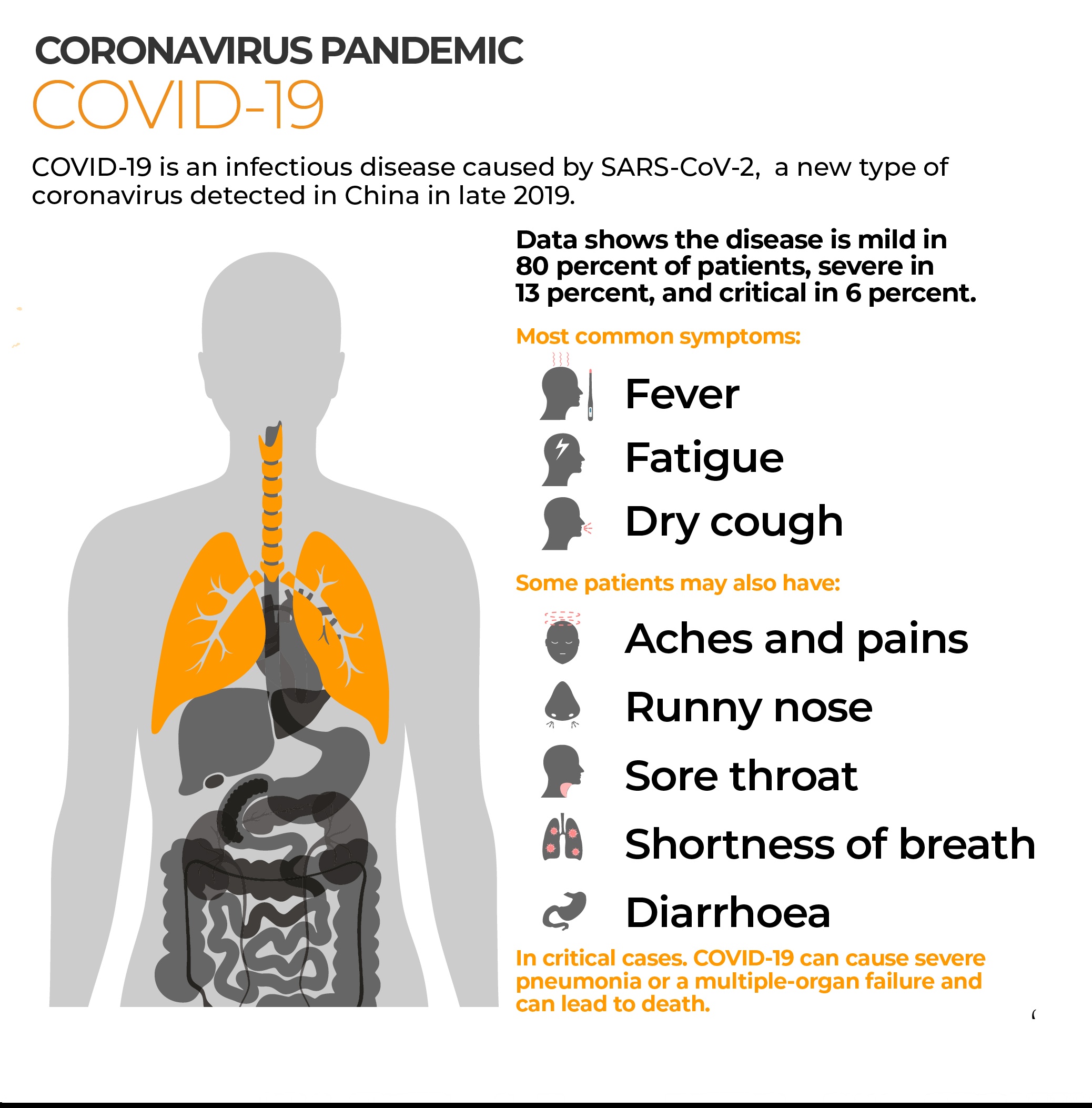
With how covid-19 has affected the world at large there has been lots of efforts by many nations to come up with working vaccines and cures for the disease. A cure would take too long to come up with so the main hope for now is looking at coming up with a viable vaccine that would provide immunity that lasts long. In this article we are going to look at the importance of vaccinations and regulations guiding the testing of vaccines in developing nations.
Importance of vaccination, this does not need much thinking into it as the benefits are wide with the main ones being stopping the spread of Covid-19 and allowing people who are at greater risks to live freely better than the current recommendations allow. A return to normalcy is another major reason that has found businesses and schools not operating to their maximum capacity but restricted in activity, a move which has hit many economies hard however necessary it was.
With over a 100 potential vaccines being tested worldwide, all at different stages of testing only 35 are undergoing clinical trials according to the World Health Organisation. This is a great positive but not one without its controversies. In April two French doctors sparked criticism for the idea of testing a potential vaccine for the Corona virus in Africa. This was viewed as racist and it has led to many people being skeptical of receiving a vaccine when it is made available. Though there should be a cause of concern from such claims, medicine professionals should understand that testing vaccines in people let alone those in developing nations is not as easy there are certain regulations that govern clinical trials research in vulnerable communities. This is done to protect vulnerable individuals from individuals who try to conduct any research that wouldn’t directly benefit the community its being done in.
The Helsinki Declaration of 1964 was the first standard for biomedical research and it stated that, the well-being of the participant was of paramount importance, recommended the use of written consent forms from all participants and all access to benefits for all participants. This was then stressed further in 1993 at the Council for International Organisations of Medical Science (CIOMS), which provided further guidelines especially for conducting clinical trials in developing countries.
The CIOMS stressed issues to do with protection of vulnerable populations, distribution of benefits and burdens among researchers and host country. From these guidelines all researchers on any clinical trial are expected to provide some form of remuneration to the host nation and participants together with ensuring that the findings of the research directly benefit the community in form of treatments and royalties that might come from the sales of treatment being tested. This is the theory of beneficence, participant must benefit from research.
The use of written consent forms as stipulated by the Helsinki Declaration follows the theory of autonomy. This means the participant has the power to opt in or opt out of a clinical trial at any stage of the trial. If a patient opts out it still remains the responsibility of the researcher to make sure participant receives proper care if it was due to any form adverse reaction. The participant’s needs outweigh those of science and the community. So for all the fear of being used as guinea pigs, people must understand that the power to receive any experimental vaccine lays with them.
RESPONSIBILITIES OF RESEARCHER
Prior to conducting research in a population or community with limited resources the researcher/sponsor should:
1) Ensure the research responds to the health needs and priorities of the target community. It is not sufficient to determine disease prevalence and that new research is needed. If successful interventions result from the research they must be made available to the community. If this is not done, the research is exploitative.
2) Ensure any product developed will be made available to the community. Before the research begins, a plan should be offered in which the clinical trial product is made available to the host nation upon completion and participants should include representatives of the nation’s government, local authorities and community members.
Agreements made by the two parties i.e. host nation and researcher should include payments, royalties, distribution costs, subsidies, technology, and intellectual property. Failure to meet the agreed upon requirements would lead to host nation suing the researcher for failure to meet agreed upon terms and termination of trials.
LEGAL REQUIREMENTS TO CONDUCT CLINICAL TRIALS
Investigators are required to seek ethics approval from all applicable IRBs/ IECs. This is to ensure that an independent body (independent from the investigators) has oversight over the ethical and scientific conduct of the study.
In Zimbabwe
1. Medical Research Council of Zimbabwe (MRCZ) – All health research
2. Institutional Review Board (IRB)/ Independent Ethics Committee (IEC) – At the institution where the research is to be conducted.
The principal investigator is required to seek approval from all applicable regulatory bodies.
In Zimbabwe
1. Medicines Control Authority of Zimbabwe (MCAZ) – All clinical trials
2. Research Council of Zimbabwe (RCZ) – Foreign researchers and/ or involving shipment of samples outside Zimbabwe
3. National Biotechnology Authority (NBA) of Zimbabwe (Study products are bio-technology based)
4. All clinical trials conducted in Zimbabwe are regulated in terms of Part III of the Medicines and Allied Substances Control Act [Chapter 15:03] and its regulations
5. No person shall conduct a clinical trial of any medicine without the prior written authorization of the Authority, granted with the approval of the Secretary of the Ministry of Health and Child Care.
6. The electronic clinical trials application platform https://e-ctr.mcaz.co.zw/
All clinical trials of vaccines and potential drugs are monitored at any stage by regulatory authorities and ethical committees, who can request information regarding the trials. This is to ensure that all this is done in accordance with Good Clinical Practices. The monitoring bodies are the MCAZ, MRCZ and IRBs.
The only African country doing clinical trials for the Covid-19 vaccine is South Africa, which is working together with the Oxford University. Since it is not recognized as part of developing nations the conduction of research is not as stringent. In the event that Zimbabwe wants to participate in these studies we can be rest assured that our ethics committees and regulatory authorities will protect the needs of the people and evaluate each candidate thoroughly before any trials commence.
Reference:
https://e-ctr.mcaz.co.zw/?fbclid=IwY2xjawMPM4xleHRuA2FlbQIxMQABHmhnPUl22cCRPZCrPB0inCkMPHWI4wPmmhNkV8hni-XVNrXqSCfSBCmsX3Bu_aem_ytV-RCueBG37Qb87LxtOVw